RF АППАРАТ ДЛЯ ЛИФТИНГАВОЗДЕЙСТВИЯ
Содержание:
Redox and electron exchanges
For many years, chemists thought of oxidation and reduction as involving
the element oxygen in some way or another. That’s where the name
oxidation came from. But they eventually learned that other elements
behave chemically in much the same way as oxygen. They decided to revise
their definition of oxidation and reduction to make it more
general—to apply to elements other than oxygen.
The second definition for oxidation and reduction is not as easy to see.
It is based on the fact that when two elements react with each other, they
do so by exchanging electrons. In an oxidation-reduction reaction like the
one above, the element that is oxidized always loses electrons. The
element that is reduced always gains electrons. The more general
definition of redox reactions, then, involves the gain and loss of
electrons rather than the gain and loss of oxygen.
In the reaction below, for example, sodium metal (Na) reacts with chlorine
gas (Cl
2
) in such a way that sodium atoms lose one electron each to chlorine
atoms:
2 Na + Cl
2
→ 2 NaCl
Because sodium loses electrons in this reaction, it is said to be
oxidized. Because chlorine gains electrons in the reaction, it is said to
be reduced.
Types of redox reactions.
Redox reactions are among the most common and most important chemical
reactions in everyday life. The great majority of those reactions can be
classified on the basis of how rapidly they occur. Combustion is an
example of a redox reaction that occurs so rapidly that noticeable heat
and light are produced. Corrosion, decay, and various biological processes
are examples of oxidation that occurs so slowly that noticeable heat and
light are
not
produced.
Combustion.
Combustion means burning. Any time a material burns, an
oxidation-reduction reaction occurs. The two equations below show what
happens when coal (which is nearly pure carbon) and gasoline (C
8
H
18
) burn. You can see that the fuel is oxidized in each case:
C + O
2
→ CO
2
2 C
8
H
18
+ 25 O
2
→ 16 CO
2
+ 18 H
2
O
In reactions such as these, oxidation occurs very rapidly and energy is
released. That energy is put to use to heat homes and buildings; to drive
automobiles, trucks, ships, airplanes, and trains; to operate industrial
processes; and for numerous other purposes.
Rust.
Most metals react with oxygen to form compounds known as oxides. Rust is
the name given to the oxide of iron and, sometimes, the oxides of other
metals. The process by which rusting occurs is also known as corrosion.
Corrosion is very much like combustion, except that it occurs much more
slowly. The equation below shows perhaps the most common form of
corrosion, the rusting of iron.
4 Fe + 3 O
2
→ 2 Fe
2
O
3
Decay.
The compounds that make up living organisms, such as plants and animals,
are very complex. They consist primarily of carbon, oxygen, and hydrogen.
A simple way to represent such compounds is to use the letters x, y, and z
to show that many atoms of carbon, hydrogen, and oxygen are present in the
compounds.
When a plant or animal dies, the organic compounds of which it is composed
begin to react with oxygen. The reaction is similar to the combustion of
gasoline shown above, but it occurs much more slowly. The process is known
as decay, and it is another example of a common oxidation-reduction
reaction. The equation below represents the decay (oxidation) of a
compound that might be found in a dead plant:
C
x
H
y
O
z
+ O
2
→ CO
2
+ H
2
O
Омоложение
С возрастом наша кожа претерпевает негативные изменения в результате влияния двух основных факторов: хронологическое старение и фотостарение.
Процессы хронологического старения необратимы. Они наступают вследствие генетических модификаций, приводящих к утончению различных слоев: эпидермиса и дермы. Кожа теряет свою эластичность.
Фотостарение наступает при изменении экспрессии генов. Это происходит из-за негативного действия факторов окружающей среды: солнце, дым, низкие температуры, ветер, неблагоприятная экологическая обстановка. Коллагеновые и эластиновые волокна деградируют. Это отражается на внешнем состоянии кожи. Она становится грубой, сухой, атоничной, морщинистой. Фотостарение может выражаться также в выпадении вен, дисхромии (цвет отклоняется от нормы).
Омолодить кожу можно на аппарате для фотоэпиляции и фототерапии Trios. Дополнительно специалисты рекомендуют пройти курс на оборудовании для электропорации Infusion.
В комплексе приборы дают результат: увлажнение, питание, отсутствие ощущения сухости.
Эффект становится заметным практически сразу же. Кожа лица — гладкая, баланс восстанавливается. Существенные улучшения наблюдаются с продолжением курса терапевтических процедур.
The Increase or Decrease in Oxidation Numbers
Laghi.l / Wikimedia Commons
Science
-
Chemistry
-
Basics
-
Chemical Laws
-
Molecules
-
Periodic Table
-
Projects & Experiments
-
Scientific Method
-
Biochemistry
-
Physical Chemistry
-
Medical Chemistry
-
Chemistry In Everyday Life
-
Famous Chemists
-
Activities for Kids
-
Abbreviations & Acronyms
-
-
Biology
-
Physics
-
Geology
-
Astronomy
-
Weather & Climate
by
Anne Marie Helmenstine, Ph.D.
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels.
Updated August 12, 2019
This is an introduction to oxidation-reduction reactions, also known as redox reactions. Learn what redox reactions are, get examples of oxidation-reduction reactions, and find out why redox reactions are important.
What Is an Oxidation-Reduction or Redox Reaction?
Any chemical reaction in which the oxidation numbers (oxidation states) of the atoms are changed is an oxidation-reduction reaction. Such reactions are also known as redox reactions, which is shorthand for reduction-oxidation reactions.
Oxidation and Reduction
Oxidation involves an increase in oxidation number, while reduction involves a decrease in oxidation number. Usually, the change in oxidation number is associated with a gain or loss of electrons, but there are some redox reactions (e.g., covalent bonding) that do not involve electron transfer. Depending on the chemical reaction, oxidation and reduction may involve any of the following for a given atom, ion, or molecule:
- Oxidation involves the loss of electrons or hydrogen OR gain of oxygen OR increase in oxidation state.
- Reduction involves the gain of electrons or hydrogen OR loss of oxygen OR decrease in oxidation state.
Example of an Oxidation-Reduction Reaction
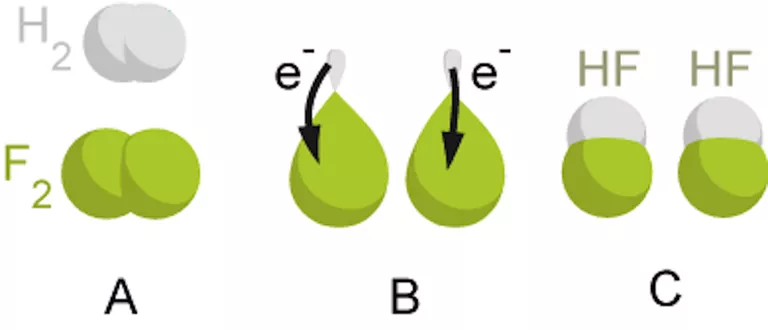
The reaction between hydrogen and fluorine is an example of an oxidation-reduction reaction:
H2 + F2 → 2 HF
The overall reaction may be written as two half-reactions:
H2 → 2 H+ + 2 e− (the oxidation reaction)
F2 + 2 e− → 2 F− (the reduction reaction)
There is no net change in charge in a redox reaction so the excess electrons in the oxidation reaction must equal the number of electrons consumed by the reduction reaction. The ions combine to form hydrogen fluoride:
H2 + F2 → 2 H+ + 2 F− → 2 HF
Importance of Redox Reactions
The electron transfer system in cells and oxidation of glucose in the human body are examples of redox reactions. Oxidation-reduction reactions are vital for biochemical reactions and industrial processes as well. Redox reactions are used to reduce ores to obtain metals, to produce electrochemical cells, to convert ammonia into nitric acid for fertilizers, and to coat compact discs.
Continue Reading